Implants replacing some of the infant’s bone with the biodegradable matrix could eliminate some of the operations currently used to treat the condition.
“The remarkable thing about this is the finding that the composition of the matrix changes what the cells around it do. Cells begin producing natural drugs to drive bone healing in direct response to the composition of the bone matrix,” said Kent Leach, professor of biomedical engineering at UC Davis.
The material is currently being tested in experiments with rats. Human trials will depend upon the success of tests in animals.
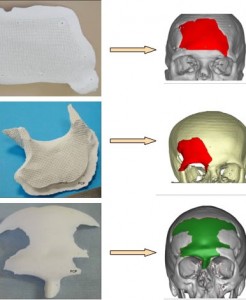
The human skull is not a smooth dome, but a patchwork of fused bones that resembles a soccer ball rather than an egg. At birth, the skull contains 45 separate pieces, joined by connective tissue, that slowly fuse together into solid bone. In most babies, this process keeps pace with brain growth, resulting in a normally shaped head.
In the standard surgery, surgeons remove fused bones, break them up and reposition some of the pieces along the edges to protect the brain. This usually slows the bone growth and allows the brain to grow.
Nevertheless, 6 to 8 percent of babies will need a second operation and 25 percent of those will need a third operation as well.
Leach believes that the environment surrounding the cells might be sending the wrong instructions, causing cells to grow wrong.
Their biodegradable implant is impregnated with stem cells from bone marrow and a synthetic version of hydroxyapatite, a chemical produced naturally in the body to stimulate bone growth.
Once implanted, bone-forming cells enter the matrix.
In their research with rats, the researchers showed dense connective tissue, suggestive of bone formation, only eight weeks after implantation.
Leach hopes that his new matrix will encourage the growth of healthy tissue and eliminate the need for second and third surgeries.
“The matrix will resorb over time, leaving only the child’s own bone,” he said.