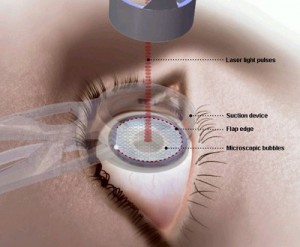
The FDA said the study to be conducted with and co-funded by the National Eye Institute and the Department of Defense will focus on laser-assisted in situ keratomileusis, a surgical procedure that uses a laser to permanently change the shape of a person’s cornea to improve their vision.
Officials said the goals of the project are to determine the percentage of patients with significant quality of life problems after undergoing LASIK and to identify predictors of such problems.
The project is composed of three phases. The objective of Phase 1, which began in July, was to design and implement a Web‑based questionnaire to assess patient-reported outcomes and evaluate quality of life issues post-LASIK.
Phase 2 will evaluate the quality of life and satisfaction following LASIK as reported by patients in a select, active duty population treated at the Navy Refractive Surgery Center. Phase 3 will be a national, multi-center clinical trial and will study LASIK’s impact on the quality of life of patients in the general pubic.
The FDA also announced it issued warning letters to 17 unidentified LASIK ambulatory surgical centers after inspections revealed inadequate adverse event reporting systems at the facilities.
About FDA
The Food and Drug Administration (FDA or USFDA) is a Government agency of the United States Department of Health and Human Services and is responsible for regulating and supervising the safety of foods, tobacco products, dietary supplements, Medication drugs, vaccines, Biopharmaceutical, blood transfusion, medical devices, Electromagnetic radiation emitting devices, veterinary products, and cosmetics. The FDA also enforces section 361 of the Public Health Service Act and the associated regulations, including sanitation requirements on interstate travel as well as specific rules for control of disease on products ranging from pet turtles to semen donations for assisted reproductive medicine techniques.
More information about https://www.fda.gov