After years of intensive research, scientists at the National Institute of Health and Johns Hopkins have been successful at safely reversing severe sickle cell disease in adults through “mini”stem cell transplantation
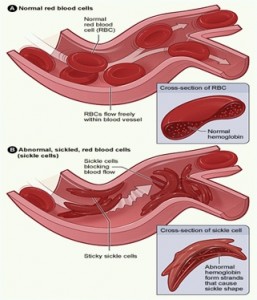
Sickle cell disease is an inherited group of genetic blood disorder characterized by abnormal crescent shaped hemoglobin. In many forms of the disease, the red blood cells change shape upon deoxygenation because of polymerization of the abnormal sickle hemoglobin .
The hemoglobin proteins stick to each other, causing the cell to get a rigid surface and sickle shape. This process damages the RBC membrane and can cause the cells to become stuck in the blood vessels. This in turn deprives the downstream tissues of oxygen and causes ischemia and infraction which may cause damage to organs like kidney, liver, spleen etc, leaving people especially children more susceptible to infection.
Symptoms often include fatigue, paleness, and shortness of breath, pain that occurs unpredictably in any body organ or joint, eye problems, yellowing of skin and eyes, delayed growth and puberty in children. Other complications include infections, stroke and acute Chest pain.
Sickle cell anemia is caused by a point mutation in the beta globin gene of hemoglobin, replacing amino acid glutamic acid with a less polar amino acid valine at the 6th position of the beta chain. It affects people who inherit two copies of an altered gene from their carrier parents. Carrier parents have a one-in-four chance of having an affected child and a one-in-two chance of having a child who is an unaffected carrier.
Carriers of sickle cell are not affected themselves, as they have a working gene as well as an altered gene.
The disease is chronic and lifelong. Individuals are most often well but their lives are punctuated by periodic painful attacks. Life expectancy is shortened with studies reporting an average age of 42 and 48 years for males and females respectively.
Various approaches are sought for preventing sickling episodes as well as for the complications of sickle-cell disease. Treatments include prophylactic antibiotics to fight infections, blood transfusions and hydroxyurea, the only drug U.S Food and Drug Administration approved for treating sickle cell disease.
Sickle cell anemia affects millions of people worldwide but is most common in people with recent ancestry in Africa, south or Central America, the Caribbean, the Mediterranean, India and the Middle East. It affects about 70,000 people in the U.S mainly African Americans and around 2 million Americans have the sickle cell trait. Statistics from the Centre for Arab Genomic Studies shows that this disease is highly prevalent in the Arab world.
Some of the world’s highest frequencies of the disease are seen in Saudi Arabia(5.2%),Oman(3.8%),Bahrain(2.1%),UAE(1.9%),Yemen(0.95%) and other Arab countries. The high prevalence of the disease has prompted extensive studies on it and almost all of the Arab countries have undertaken vast amounts of work on clinical features, genetics and management of sickle cell anemia.
STRATEGIES TO FIND A CURE.
Researchers have adopted a number of approaches to find a cure for this disease. In an early study in 2007, scientists from the Whitehead Institute for Biomedical Research in Cambridge and the University of Alabama at Birmingham were successful in curing sickle cell anemia in mice with the help of induced pluripotent stem cells. Cells were extracted from the skin of mice and four dormant genes that are active in days-old embryo were turned on in them. This transformed the cells into an embryonic state(iPS cells).Then they corrected the genetic flaw that causes sickle cell anemia by genetic engineering. The iPS cells were then grown into bone marrow stem cells by exposing them to special growth factors and culture conditions. These bone marrow stem cells were reinjected into the mice. Weeks later the mice produced the normal version of hemoglobin beta protein and were cured of the disease. But this technique is far from perfected to be used in humans.
In previous studies, a gene called BCL11A was found to be involved in blocking the expression of fetal hemoglobin in adults. In the embryonic mice, inactivation of the BCL11A gene led to a robust expression of gamma-globin (the fetal form of hemoglobin) during late gestation: more than 90 percent of the globin produced was of this type.
Tissue-specific deletion of the BCL11A gene in the adult mice (8-10 weeks old) resulted in an increase of more than 1,000-fold in gamma-globin gene expression in the bone marrow erythroblasts (the precursors to red blood cells) of the experimental mice in comparison to control mice. This increase in the gamma-globin expression after inactivation of BCL11A was rapid and persisted during the course of the experiments (up until the mice were 25 weeks old). This research opens up a new avenue for treatment, a way to genetically activate healthy fetal hemoglobin in the red blood cells of patients with these lifelong blood disorders.
A more promising approach that has yielded successful results recently involves “mini” stem cell transplantation. The study was conducted by scientists at the National Institute of Health and John Hopkins who were able to reverse severe sickle cell disease in adults. Adults have typically not been candidates because they were thought to be too sick to handle the high doses of chemotherapy and radiation necessary to prepare the body for the procedure. Until now, transplantation was generally reserved for more resilient children, whose bodies had not yet suffered as much damage from sickle cell disease. But the new method allows for a less grueling pre-transplantation routine, one that even adults with severe sickle cell disease can tolerate.
The trials were done on 10 patients with severe sickle cell disease who received intravenous transplants of blood forming stem cells. The transplanted stem cells came from peripheral blood of healthy related donors matched to the patient’s tissue type. All patients in the study ranging from 16 to 45 years were treated with what researchers call “mini” transplant along with an immune suppressive drug called rapamycin.
A “minitransplant” uses lower, less toxic, doses of chemotherapy to prepare the patient for an allogeneic transplant. The use of low doses eliminates some, but not all, of the patient’s bone marrow. It also suppresses the patient’s immune system to prevent rejection of the transplant. Traditional bone marrow transplant (BMT) or peripheral blood stem cell transplant (PBSCT) used high doses of chemotherapy to wipe out the immune system before the transplanted cells are injected, a process that has many side effects including serious bacterial and fungal infections and also affects fertility.
Unlike this, bone marrow cells from both the donor and the patient may exist in the patient’s body for some time after a “minitransplant.”Using this procedure nine of 10 patients treated have normal red blood cells and reversal of organ damage caused by the disease. None of the patients experienced graft-versus-host disease, one of the most common and potentially fatal complications of BMT,in which the body rejects the new bone marrow.
The NIH/Johns Hopkins team is conducting further studies on immune cells gathered from patients in the study and looking at a combination of rapamycin with a well known cancer drug cyclophosphamide.Other teams at the Hopkins are studying the use of half matched donors, helping to widen the pool of potential donors for stem cell transplantation.
Currently, there are only a limited number of therapies available for patients with sickle cell disease and thalassemia, another disorder involving abnormal hemoglobin. More research needs to be devoted in this area to save the lives of millions of children and adults who suffer from the painful and serious complications of this fatal disease.