 The most controversial and most prized Tissue grown from human embryonic stem cells grown in a lab, could at last make their way into the human body.
The most controversial and most prized Tissue grown from human embryonic stem cells grown in a lab, could at last make their way into the human body.
After a decade of scientific and political discussions,doubts and worries, several therapies are now edging towards human trials. Which will be first? that is the question.
A decade ago the medical possibilities seemed limitless but now it is clear that human embryonic stem cells are unique in their ability to grow into all 200 types of human tissue. They are unique in their ability to turn into every type of cell in the body
The idea was to create a range of hESC lines and to implant into people tissues and organs derived from the lines that best matched their existing tissue to prevent immune rejection.
 A big hurdle in this new research came in 2001 in the US when then-president George Bush restricted federally funded researchers to working on just a dozen or so hESC lines, to please those who object to hESC research on religious or ethical grounds. Research continued in other rich countries, however, and President Barack Obama has now overturned the Bush restrictions.
A big hurdle in this new research came in 2001 in the US when then-president George Bush restricted federally funded researchers to working on just a dozen or so hESC lines, to please those who object to hESC research on religious or ethical grounds. Research continued in other rich countries, however, and President Barack Obama has now overturned the Bush restrictions.
But another challenge looms: stem cells’ prized versatility makes their behaviour in the body unpredictable. This has led regulatory bodies like the US Food and Drug Administration (FDA) to view tissue created from hESCs with extreme caution. If a few hESCs fail to differentiate into the desired tissue they may move away from the target site and disrupt other bodily processes or turn cancerous.
Back in January, it looked likely that biotech firm Geron of Menlo Park, California, would be first to implant hESC-derived neural cells into the human body when it won FDA approval to treat people with spinal cord injuries. But the trial is now on hold after cysts formed in some treated animals.
 Now, although Geron says it hopes to implant its neural cells into people by September 2010, it could be pipped to the post. Last week, Advanced Cell Technology of Worcester, Massachusetts, applied to the FDA for permission to treat a rare inherited eye condition with cells grown from hESCs.
Now, although Geron says it hopes to implant its neural cells into people by September 2010, it could be pipped to the post. Last week, Advanced Cell Technology of Worcester, Massachusetts, applied to the FDA for permission to treat a rare inherited eye condition with cells grown from hESCs.
ACT chief scientific officer Robert Lanza cites several reasons why these cells might raise fewer safety concerns than Geron’s therapy (see opposite), as does a French team planning to apply next year to treat people who have burns with layers of human skin made from hESCs. Treatments for diabetes and heart disease are also on the horizon.
Evan Snyder of the Burnham Institute in La Jolla, California, an adviser to the FDA on stem cells, says that the FDA may, justifiably, demand an “unachievable” level of assurance over the safety of stem cells. Alan Colman, a stem cell pioneer at the Institute of Medical Biology in Singapore, is more hopeful. “I think the ACT target of the eye is a much better one than the Geron one,” he says. “Safety issues, while still there, are tractable.”
Eyes
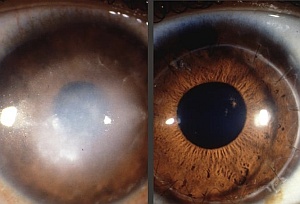 The first human embryonic stem cells (hESCs) to make it into the human body could be ones that save sight. Last week, Advanced Cell Technology (ACT) of Worcester, Massachusetts, applied for permission to inject stem-cell derived retinal pigment epithelial cells (RPEs) into the eyes of 12 patients with Stargardt’s macular dystrophy, a rare inherited condition which leads to blindness in middle age.
The first human embryonic stem cells (hESCs) to make it into the human body could be ones that save sight. Last week, Advanced Cell Technology (ACT) of Worcester, Massachusetts, applied for permission to inject stem-cell derived retinal pigment epithelial cells (RPEs) into the eyes of 12 patients with Stargardt’s macular dystrophy, a rare inherited condition which leads to blindness in middle age.
RPEs form a layer of cells beneath the photoreceptor cells of the retina where they provide nourishment, recycle pigments, and dispose of debris. In people with Stargardt’s, RPEs deteriorate and die off. Robert Lanza of ACT says the sight of rodents with the equivalent of Stargardt’s returned to “nearly normal” after injection of RPEs. He says that cells implanted in the eye are less likely to stray because they are not connected to the rest of the body via the bloodstream. It is also easier to track and remove cells from the eye, he claims.
hESC-derived RPEs are also being developed in the UK. By 2011 they could be used in those who’ve lost sight due to age-related macular degeneration, says team leader Pete Coffey of University College London.
Skin
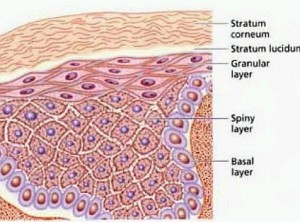 Last week came the first report of human skin made from hESCs. Although the cells have so far only been tested on mice, the idea is to use skin grown from these stem cells as temporary grafts for people with burns, while they wait for a permanent graft to be grown from their own tissue.
Last week came the first report of human skin made from hESCs. Although the cells have so far only been tested on mice, the idea is to use skin grown from these stem cells as temporary grafts for people with burns, while they wait for a permanent graft to be grown from their own tissue.
It’s a relatively new suggestion – but it could steal a march on the others because it is a temporary fix. It is also easy to monitor visually, and can easily be removed if problems occur.
In the recent study, Christine Baldeschi, together with a team of researchers at the Institute for Stem Cell Therapy and Exploration of Monogenic Diseases in Evry, France, used hESCs to make patches of keratinocytes, cells that mature into skin. They attached these to the skin of mice, where the cells grew into all five layers of the epidermis, the upper surface of human skin (The Lancet, DOI: 10.1016/S0140-6736(09)61496-3). “We’re planning a human trial, and hope to apply within a year to the French authorities for permission to go ahead,” she says. “If it works well, we may apply to use it in permanent grafts.”
Pancreas
The task of turning hESCs into insulin-producing beta cells that can treat diabetes now falls to just two firms.
Out in front is Novocell of San Diego, California. Following successful experiments in animals, it now plans to put the cells in an implantable device that lets in oxygen, nutrients and hormones, but stops the cells from escaping and isolates them from the body’s immune system. “Because the implanted device will be located just below the skin, monitoring the cells and retrieving them if necessary is possible,” says a company spokesperson. This would make it safer than if the cells were dispersed randomly in the body.
Biotech firm Geron has also developed islet-like cells from hESCs for treating diabetes.
Spine
Back in January it looked certain that neural stem cells would be the first cells derived from hESCs to be implanted in humans. Following a slew of rejected applications, the biotech firm Geron finally received permission from the FDA to implant these into people paralysed by severe injuries to the lower spine.
In August, the FDA put the trial on hold. Geron later revealed that the FDA was investigating the appearance of cysts in some treated animals. The firm also said that the cysts didn’t grow or spread beyond the injury sites, nor appear to harm the animals.
It’s now identifying cellular markers so that rogue cells forming the cysts can be screened out in future. It hopes that the trial will get going by September next year, and has applied for it to be extended to patients with neck injuries. The company is also exploring other applications for the cells, including multiple sclerosis, stroke and Alzheimer’s disease, says a spokesperson.
Heart
Philippe Menasché and his colleagues at the George Pompidou Hospital in Paris, France, hope to start repairing damaged hearts with cells made from hESCs by 2012.
When they injected their stem cell-derived cardiac progenitor cells into the hearts of animals after heart attacks, the cells matured into beating heart cells, which helped repair the damaged tissue.
The aim now is to inject the cells into people who have had a coronary artery bypass operation whose own tissue has been damaged. “We plan to apply within the next two years,” says Menasché. “But we will not rush, and remain cautious about whether it will work.” Biotech firm Geron, too, is developing cardiomyocytes derived from hESCs to treat failing hearts.
Article Published by New Scientis Copyrights New Scientis. Orignal article link. https://www.newscientist.com/article/mg20427364.400-race-is-on-to-use-embryonic-stem-cells-in-humans.html?full=true
