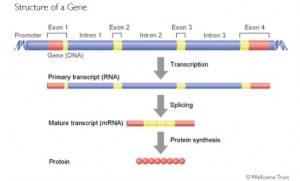
Genes are composed of meaningful sequences, called exons which code for proteins, separated by non coding regions called introns. In order for cells to produce RNA – the material that is required to create proteins that are vital for life – they must precisely remove meaningless introns and bind meaningful exons together, a process called gene “splicing.”
How cells differentiate between what’s useful and what’s not essential in our complicated and messy genetic code is a big mystery – one with extremely important implications. Now, Prof. Gil Ast and his doctoral student Schraga Schwartz at the Sackler School of Medicine at Tel Aviv University are successfully finding answers.
Their groundbreaking findings, recently published in Nature Structural and Molecular Biology, reveal a new mechanism to explain how splicing works. They’ve discovered that the structure of DNA itself affects the ways RNA is spliced. “These findings,” says Prof. Ast, “will bring us closer to understanding diseases like cystic fibrosis and certain forms of cancer that result from our cells’ failure to edit sequences properly.”
Rewriting textbook science on DNA
Until now, how RNA was “edited” to fit together has been a mystery. The TAU revelations provide important information about creating proteins, and give new clues to drug developers to better understand how diseases such as cancer and genetic disorders operate at the gene level. That insight can offer significant new cellular mechanisms to create innovative drug therapies.
“We’ve found something previously unknown,” Prof. Ast explains. “At the DNA level, exons are packaged differently than introns. This fact is significant, telling us a process of gene expression is taking place at an earlier step than previously believed.” This can give new clues to scientists seeking to detect and diagnose diseases before they erupt, he believes.
Take cancer, for example. In cancerous cells especially, DNA itself is structured differently than in non-cancer cells. These structures may change the way RNA is edited, leading to different joining together of exons, and therefore to different proteins, explains Prof. Ast. His lab is concurrently investigating new drug platforms to take advantage of this new biological discovery which could lead to an entirely new class of drugs.
New hope for rare genetic diseases
“We’ve been working on a compound, and are trying to understand how these structural marks vary between normal and cancer cells. If we can understand how the processing of RNA is different in diseased cells, we will hopefully find something that can change it,” he explains.
In rare and common genetic disorders, and diseases like cancer, there are different ways in which the DNA machinery produces non-functioning or damaging proteins. Genes are made from exons, but not all exons are necessarily used to produce mature RNA, Prof. Ast explains.
Sometimes genes might skip an exon for mysterious reasons: “If you skip at the wrong place, this will lead to the production of a non-productive, or even damaging, protein instead of normal proteins. This is like building a skyscraper with faulty steel beams. A big no-no.”
Prof. Ast’s new observations have shown that many cellular mutations are changing the gene splicing mechanism, an area that should be targeted in drug development. If drugs can target the mechanism that causes diseases, he hopes they will be able to halt the progression of the disease.
Source: George Hunka
American Friends of Tel Aviv University./